aqueous iron chloride and sodium carbonate solution yields aqueous sodium chloride and a precipitate of iron - Brainly.in

Chemical reaction-Rusty red iron(III) hydroxide precipitate (Fe(OH)3) in test tube formed reaction between iron(III) chloride (FeCl3) & sodium carbonate (Na2CO3): FeCl3+Na2CO3+H2O ->Fe(OH)3+NaCl+CO2 Photos | Adobe Stock
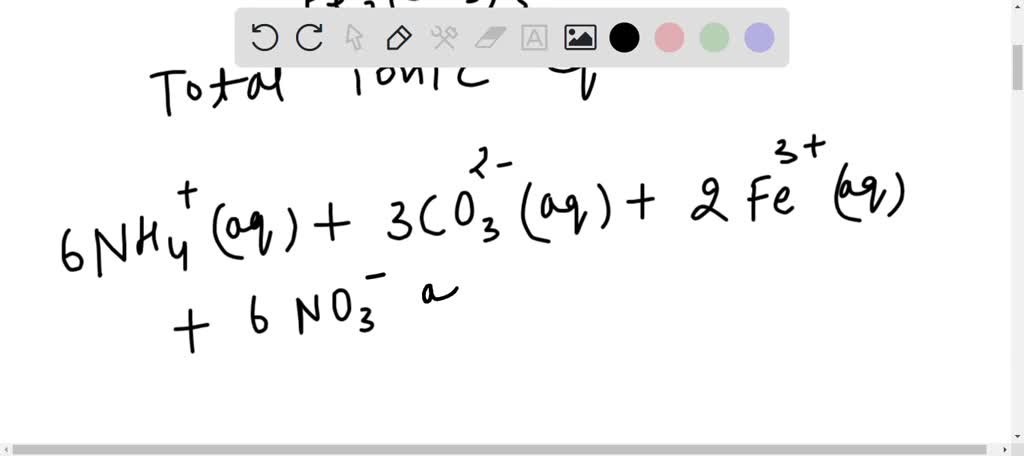
SOLVED: Ammonium carbonate and iron(iii) nitrate are combined, solid iron( iii) carbonate and a solution of ammonium nitrate are formed. the net ionic equation for this reaction is: